Covid-19 antiviral pill will be rolled out at selected polyclinics and PHPCs in phases: MOH
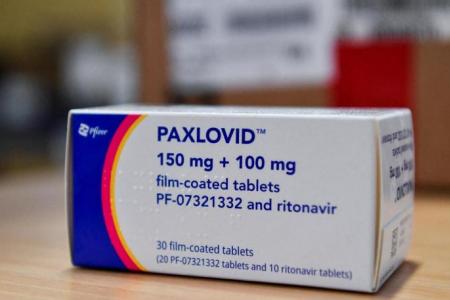
Paxlovid, a new antiviral pill to treat Covid-19, will be rolled out at selected polyclinics and Public Health Preparedness Clinics (PHPCs) in phases to patients to reduce the likelihood of hospitalisation.
This is the first Covid pill approved by the Health Sciences Authority (HSA) for use here. Currently, treatment is given based on symptoms such as cough, fever and sore throat.
The Ministry of Health (MOH) told The Straits Times that patients at polyclinics and PHPCs who are at risk of severe Covid-19 and who are within five days of onset of illness will be eligible for the drug. It did not specify when it will be rolled out.
Eligible patients at both public and private hospitals who are at highest risk of severe illness can also receive the drug.
The drug has to be taken twice daily for five days, and should be given as soon as possible within five days of the onset of symptoms.
MOH said that it will fully cover the costs of Paxlovid for use in the eligible primary care settings once it is rolled out there.
The drug has cost the US government about US$530 (S$710) for a five-day course.
"The main intention is to reduce the likelihood of high risk patients developing severe Covid-19 and requiring hospital admission, particularly during this period where the number of cases currently hospitalised remains high and healthcare workers are still under significant pressure," MOH added.
These include patients who are immunocompromised, elderly, and those with active cancer, severe lung, heart or kidney disease.
The National Centre for Infectious Diseases (NCID) told ST that it has begun administering the drug to "a handful" of patients two weeks ago, and that it is already being used in acute hospitals.
MOH said there are strict guidelines governing its use.
It added that it has worked with NCID to put in place monitoring processes to ensure that the drug is prescribed in accordance with prevailing guidance.
HSA said on Feb 3 that it has reviewed available clinical data for the drug and found that it could reduce Covid-19-related hospitalisation or death by 88.9 per cent when given within three days from the onset of symptoms.
The efficacy rate was 87.8 per cent when given within five days of symptoms appearing.
HSA also noted during Pfizer's clinical study there were only few instances of side effects.
Common side effects reported were mild to moderate, such as altered sense of taste, diarrhoea, vomiting, hypertension, muscle pain and chills.
Singapore is not the first country to be rolling out the drug in the community.
Canada cleared it for use in January this year.
In Britain, the government's medical guidance advises that patients who are hospitalised for Covid-19 befirst offered Paxlovid, followed by antiviral drug remdesivir if Paxlovidis unsuitable.
Paxlovid is also the recommended first-line treatment for eligible Covid-19 patients in the community. It was approved in Britain in December last year.
Another antiviral pill, molnupiravir, is currently being assessed by HSA for use here.
MOH had earlier signed a supply agreement with MSD Pharma -known as Merck in the United States and Canada - to purchase the drug.
The drug is also targeted at those who are at risk of developing severe illness, and it is said to be most effective when given early in the course of infection.
However, the Institute for Clinical and Economic Review, a US-based drug pricing research organisation, said that in clinical trials, molnupiravir was only able to cut hospitalisation rates by 30 per cent.
The US Food and Drug Administration has said that while Merck's experimental Covid-19 antiviral pill appears effective, it may pose risks for pregnant women, including birth defects and toxicity to developing fetuses.
Get The New Paper on your phone with the free TNP app. Download from the Apple App Store or Google Play Store now