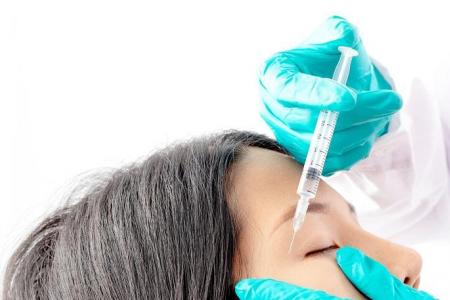
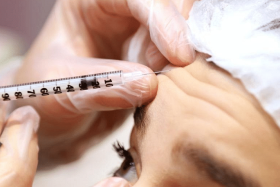
Woman goes blind after dermal filler aesthetic treatment; first reported case in S’pore
In the first such case of its kind in Singapore, a woman went blind in July after she was administered a dermal filler that temporarily reduces wrinkles and folds in the skin.
She was injected with the dermal filler AestheFill at a clinic in Redhill.
Parvus, the Singapore distributor of AestheFill, confirmed the incident and said it was in direct contact with all concerned parties and “working diligently to understand the circumstances” surrounding it.
The Health Sciences Authority (HSA), which regulates health products, told The Straits Times that it is investigating whether there were batch-related defects that could have affected product safety and quality. AestheFill was registered for use in Singapore in October 2021.
An HSA spokesman said that on July 29, Parvus reported an incident of blood vessel occlusion – or blockage – leading to blindness after the administration of AestheFill.
“Locally, this is the first adverse event report for blindness resulting from dermal fillers,” she said.
HSA classifies dermal fillers as Class D medical devices, which carry the highest risk, she added.
Companies are required to report such adverse events to HSA within 10 days.
ST’s repeated attempts to contact the doctor who carried out the procedure were unsuccessful.
His clinic’s website and social media page point to his proficiency in the domain. He is a certified trainer in AestheFill injections.
Parvus general manager Brendan Pang told ST that only qualified healthcare professionals can administer AestheFill.
Since receiving approval from HSA on Oct 1, 2021, it has been widely used by aesthetic practitioners in Singapore, said Mr Pang.
“It is an internationally recognised filler for aesthetic treatments. As with any medical procedure, there are inherent risks, which are fully communicated to patients by the trained medical doctors prior to treatment,” he added.
“Our company continuously invests in training programmes focused on injection anatomy and technique upgrades to ensure optimal patient outcomes.”
Sources with knowledge of the incident described the patient as young and married.
She apparently suffered sudden blindness after she was injected with AestheFill at the clinic. It is understood to have affected both her eyes.
The HSA spokesman said, however, that there has been no noticeable increase in reports of adverse events for aesthetic implants such as dermal fillers in Singapore.
Even so, blindness due to blood vessel blockage is a known risk for dermal fillers, she said.
Such risks are typically explained in the instructions for use given to healthcare practitioners. The instructions for AestheFill caution against injecting into blood vessels, as it may cause blockage.
Other common complications that may arise from the use of dermal fillers include swelling, redness, raised bumps in or under the skin (nodules or granulomas), skin blanching or the paling of skin (associated with injection into blood vessels), as well as the temporary blurring of vision, said the HSA spokesman.
Clinicians administering dermal fillers such as AestheFill are required to undergo training by the respective companies, she added.
“Consumers are advised to discuss with their clinician the risks and suitability of the dermal fillers before going for the procedure,” she said.
A study published in the BMC Ophthalmology online medical journal in March documented the case of a 23-year-old woman in Taiwan experiencing sudden blindness in one eye after an AestheFill injection.
There were other instances in Taiwan (2017) and Australia (2018), where individuals reportedly lost their vision due to dermal filler injections.
According to a 2018 report by Australian investigative show Four Corners, about 100 people had reportedly gone blind from filler injections worldwide.
The price of a dermal filler in Singapore typically starts from about $700.
The frequency of administration varies based on the area to which it is applied.
For example, nose fillers can be done once a year, while those on the lips can be administered every six to nine months.
Get The New Paper on your phone with the free TNP app. Download from the Apple App Store or Google Play Store now